
SL Paper 1
What is the value of x when 32.2 g of Na2SO4•xH2O are heated leaving 14.2 g of anhydrous Na2SO4? Mr(H2O) = 18; Mr(Na2SO4) = 142.
Na2SO4•xH2O (s) → Na2SO4 (s) + xH2O (g)
A. 0.1
B. 1
C. 5
D. 10
Markscheme
D
Examiners report
What is the molecular formula of a hydrocarbon containing 84.6% carbon by mass with a molar mass of 142.3 g mol−1?
A. C20H44
B. C11H10
C. C10H22
D. C5H11
Markscheme
C
Examiners report
What is the sum of the integer coefficients when propene undergoes complete combustion?
__C3H6 (g) + __O2 (g) → __CO2 (g) + __H2O (l)
A. 11
B. 17
C. 21
D. 23
Markscheme
D
Examiners report
Very well answered. 88 % of the candidates balanced the equation and added up the integer coefficients correctly.
Which is correct?
A. Mixtures are either homogeneous or heterogeneous and their chemical properties are an average of the individual component properties.
B. Mixtures are never heterogeneous and their chemical properties are an average of the individual component properties.
C. Mixtures are either homogeneous or heterogeneous and the components retain their individual chemical properties.
D. Mixtures are never homogeneous and the components retain their individual chemical properties.
Markscheme
C
Examiners report
How many moles of oxygen atoms are there in 0.500 mol of hydrated iron(II) ammonium sulfate, (NH4)2Fe(SO4)2•6H2O(s)?
A. 4.00
B. 7.00
C. 8.00
D. 14.00
Markscheme
B
Examiners report
What is the molar mass, in , of a compound if of the compound has a mass of ?
A.
B.
C.
D.
Markscheme
A
Examiners report
70% of candidates correctly found the molar mass of a substance given the mass of 0.200 mol. Good to see that very few were fooled by the same answer but incorrect significant figures.
What is the sum of the coefficients when the following equation is balanced using the smallest whole numbers?
__C6H12O6 (aq) → __C2H5OH (aq) + __CO2 (g)
A. 4
B. 5
C. 9
D. 10
Markscheme
B
Examiners report
Which contains the greatest number of moles of oxygen atoms?
A. 0.05 mol Mg(NO3)2
B. 0.05 mol C6H4(NO2)2
C. 0.1 mol H2O
D. 0.1 mol NO2
Markscheme
A
Examiners report
0.20 mol of magnesium is mixed with 0.10 mol of hydrochloric acid.
Which is correct?
Markscheme
C
Examiners report
What is the sum of the coefficients when the equation is balanced with the smallest whole numbers?
__BaCl2 (aq) + __Fe2(SO4)3 (aq) → __FeCl3 (aq) + __BaSO4 (s)
A. 4
B. 6
C. 8
D. 9
Markscheme
D
Examiners report
This question was well answered while one teacher commented that using 1 as a coefficient in a “sum the coefficients” question seemed tricky.
Which statements about mixtures are correct?
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
B
Examiners report
What is the sum of the coefficients when the equation is balanced with whole numbers?
__MnO2 (s) + __HCl (aq) → __MnCl2 (aq) + __H2O (l) + __Cl2 (g)
A. 6
B. 7
C. 8
D. 9
Markscheme
D
Examiners report
Which statement describes all homogeneous mixtures?
A. Any sample has the same ratio of the components.
B. The components are covalently bonded together.
C. The components cannot be easily separated.
D. The mixture needs a specific ratio of components to form.
Markscheme
A
Examiners report
What is the number of hydrogen atoms in 2.00 moles of Ca(HCO3)2?
Avogadro’s constant, L or NA: 6.02 × 1023 mol−1
A. 2.00
B. 4.00
C. 1.20 × 1024
D. 2.41 × 1024
Markscheme
D
Examiners report
8.8 g of an oxide of nitrogen contains 3.2 g of oxygen. What is the empirical formula of the compound?
A. N2O5
B. N2O
C. NO2
D. NO
Markscheme
B
Examiners report
Which is a homogeneous mixture?
A. Oil and water
B. Sand and water
C. Ethanol and water
D. Chalk and sand
Markscheme
C
Examiners report
What is the maximum volume, in dm3, of CO2(g) produced when 1.00 g of CaCO3(s) reacts with 20.0 cm3 of 2.00 moldm–3 HCl(aq)?
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
Molar volume of gas = 22.7 dm3mol–1; Mr(CaCO3) = 100.00
A.
B.
C.
D.
Markscheme
C
Examiners report
0.2 mol of sodium hydrogencarbonate is decomposed by heating until constant mass.
2 NaHCO3 (s) → Na2CO3 (s) + H2O (g) + CO2 (g)
How many moles of gas are produced?
A. 0.1
B. 0.2
C. 0.3
D. 0.4
Markscheme
B
Examiners report
What is the percentage yield when 7 g of ethene produces 6 g of ethanol?
Mr(ethene) = 28 and Mr(ethanol) = 46
C2H4(g) + H2O(g) → C2H5OH(g)
A.
B.
C.
D.
Markscheme
D
Examiners report
Which factors affect the molar volume of an ideal gas?
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
A
Examiners report
Which volume of ethane gas, in , will produce of carbon dioxide gas when mixed with of oxygen gas, assuming the reaction goes to completion?
A.
B.
C.
D.
Markscheme
B
Examiners report
More than 65% of candidates were able to apply coefficient ratios in a stoichiometry question involving only gaseous reactants and products.
Which contains the most atoms of oxygen?
A. 64 g of O2
B. 1.2 × 1024 molecules of O2
C. 64 g of C3H5O3
D. 1.2 × 1024 molecules of C3H5O3
Markscheme
D
Examiners report
Which molecule has the same empirical formula as molecular formula?
A. CH3COOH
B. C2H5OH
C. C2H4
D. C4H10
Markscheme
B
Examiners report
Which sample contains the fewest moles of HCl?
NA = 6.02 × 1023 mol–1.
Molar volume of an ideal gas at STP = 22.7 dm3 mol–1.
A. 10.0 cm3 of 0.1 mol dm–3 HCl (aq)
B. 6.02 × 1024 molecules of HCl (g)
C. 0.365 g of HCl (g)
D. 2.27 dm3 of HCl (g) at STP
Markscheme
A
Examiners report
Which equation represents the deposition of iodine?
A. I2 (g) → I2 (l)
B. I2 (g) → I2 (s)
C. I2 (l) → I2 (g)
D. I2 (s) → I2 (g)
Markscheme
B
Examiners report
58% of the candidates identified the equation that represented the deposition of iodine. The most commonly chosen distractor was the condensation of gaseous iodine. Sublimation was also quite commonly chosen.
What is the volume, in cm3, of the final solution if 100 cm3 of a solution containing 1.42 g of sodium sulfate, Na2SO4, is diluted to the concentration of 0.020 mol dm–3?
Mr(Na2SO4) = 142
A. 50
B. 400
C. 500
D. 600
Markscheme
C
Examiners report
What is the sum of the coefficients when the equation is balanced with whole numbers?
__Sn(OH)4 (aq) + __NaOH (aq) → __Na2SnO3 (aq) + __H2O (l)
A. 4
B. 5
C. 6
D. 7
Markscheme
D
Examiners report
5.0 cm3 of 2.00 moldm–3 sodium carbonate solution, Na2CO3(aq), was added to a volumetric flask and the volume was made up to 500 cm3 with water. What is the concentration, in moldm–3, of the solution?
A. 0.0050
B. 0.0040
C. 0.020
D. 0.010
Markscheme
C
Examiners report
Which amount, in mol, of sodium chloride is needed to make 250 cm3 of 0.10 mol dm−3 solution?
A. 4.0 × 10−4
B. 0.025
C. 0.40
D. 25
Markscheme
B
Examiners report
Which diagram represents a heterogeneous mixture?
Markscheme
A
Examiners report
A few G2 forms suggested that candidates could have chosen A or B. However, homogeneous and heterogeneous mixtures are usually represented in such a way while only option A shows a clear separation in the mixture. We will avoid schematic diagrams in the future for this type of questions.
16 g of bromine react with 5.2 g of metal, M, to form MBr2. What is the relative atomic mass of the metal M? (Ar : Br = 80)
A. 13
B. 26
C. 52
D. 104
Markscheme
C
Examiners report
What is the number of atoms of oxygen in 2.0 mol of hydrated sodium carbonate, Na2CO3•10H2O? Avogadro’s constant, L or NA: 6.02 × 1023 mol–1
A. 6
B. 26
C. 3.6 × 1024
D. 1.6 × 1025
Markscheme
D
Examiners report
Which of these molecular formulae are also empirical formulae?
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
B
Examiners report
One of the best answered questions on paper 1. Very few of the candidates struggled with distinguishing molecular and empirical formula.
How many grams of sodium azide, NaN3, are needed to produce 68.1 dm3 of N2 (g) at STP?
Molar volume at STP = 22.7 dm3 mol–1; Mr(NaN3) = 65.0
2NaN3 (s) → 3N2 (g) + 2Na (s)
A. 32.5
B. 65.0
C. 130.0
D. 195.0
Markscheme
C
Examiners report
Which graph would not show a linear relationship for a fixed mass of an ideal gas with all other variables constant?
A. P against V
B. P against
C. P against T
D. V against T
Markscheme
A
Examiners report
The idea of P vs 1/V as being non-linear was the most common mistake, with just over 50 % of candidates earning this mark.
30 g of an organic compound produces 44 g CO2 and 18 g H2O as the only combustion products. Which of the following is the empirical formula for this compound?
Mr CO2 = 44 Mr H2O = 18
A. CH2
B. CH3
C. CHO
D. CH2O
Markscheme
D
Examiners report
63% of the candidates determined the empirical formula of the organic compound correctly using the masses of the sample and the combustion products.
What is the sum of the coefficients when the equation is balanced with the lowest whole number ratio?
__Na2S2O3(aq) + __HCl(aq) → __S(s) + __SO2(g) + __NaCl(aq) + __H2O(l)
A. 6
B. 7
C. 8
D. 9
Markscheme
C
Examiners report
What is the concentration, in mol dm−3, of 20.0 g of NaOH (Mr = 40.0) in 500.0 cm3?
A. 0.250
B. 0.500
C. 1.00
D. 4.00
Markscheme
C
Examiners report
75 % of the candidates were able to calculate the molar concentration of the NaOH solution. The question had a good discrimination index.
What is the volume of gas when the pressure on 100 cm3 of gas is changed from 400 kPa to 200 kPa at constant temperature?
A. 50.0 cm3
B. 100 cm3
C. 200 cm3
D. 800 cm3
Markscheme
C
Examiners report
80 % of the candidates were able to deduce the new volume of a sample of gas after the pressure was halved. The most commonly chosen distractor (A) was the value that assumed a direct proportionality between volume and pressure.
Which compound has the greatest percentage by mass of nitrogen atoms?
A. N2H4
B. NH3
C. N2O4
D. NaNO3
Markscheme
A
Examiners report
The two containers shown are connected by a valve. What is the total pressure after the valve is opened and the two gas samples are allowed to mix at constant temperature?
A. 1.5 × 105 Pa
B. 2.3 × 105 Pa
C. 2.5 × 105 Pa
D. 5.0 × 105 Pa
Markscheme
B
Examiners report
What is the empirical formula of a hydrocarbon with 75 % carbon and 25 % hydrogen by mass?
A. C3H
B. CH2
C. C2H6
D. CH4
Markscheme
D
Examiners report
Well answered and straight forward.
What is the sum of the coefficients when the equation is balanced with whole numbers?
—C8H18(g) + —O2(g) → —CO(g) + —H2O(l)
A. 26.5
B. 30
C. 53
D. 61
Markscheme
C
Examiners report
What is the molecular formula of a compound with an empirical formula of CHO2 and a relative molecular mass of 90?
A. CHO2
B. C2H2O4
C. C3H6O3
D. C4H10O2
Markscheme
B
Examiners report
Which volume, in cm3, of 0.20 mol dm-3 NaOH (aq) is needed to neutralize 0.050 mol of H2S(g)?
H2S(g) + 2NaOH(aq) → Na2S(aq) + 2H2O(l)
A. 0.25
B. 0.50
C. 250
D. 500
Markscheme
D
Examiners report
How many moles of magnesium hydroxide are produced with 0.50 mol of ammonia?
Mg3N2 (s) + 6H2O (l) → 3Mg(OH)2 (aq) + 2NH3 (aq)
A. 0.25
B. 0.33
C. 0.75
D. 1.5
Markscheme
C
Examiners report
This was one of the easier questions on the paper. 79 % of the candidates were able to deduce the amount of a product given the amount of another product and the balanced equation.
What is the expression for the volume of hydrogen gas, in dm3, produced at STP when 0.30 g of magnesium reacts with excess hydrochloric acid solution?
Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
Molar volume of an ideal gas at STP = 22.7 dm3mol−1
A.
B.
C.
D.
Markscheme
B
Examiners report
What volume of oxygen, in dm3 at STP, is needed when 5.8 g of butane undergoes complete combustion?
A.
B.
C.
D.
Markscheme
B
Examiners report
What is the concentration of chloride ions, in mol dm−3, in a solution formed by mixing 200 cm3 of 1 mol dm−3 HCl with 200 cm3 of 5 mol dm−3 NaCl?
A. 1
B. 2
C. 3
D. 6
Markscheme
C
Examiners report
52% of the candidates calculated the concentration of chloride ions in the titration. The distractors were chosen almost equally.
What is the resulting concentration, in mol dm−3, when 1.0 cm3 of 0.500 mol dm−3 nitric acid solution is diluted to 50.0 cm3 with water?
A. 0.002
B. 0.01
C. 0.04
D. 0.1
Markscheme
B
Examiners report
The volume of a sample of gas measured at 27 °C is 10.0 dm3. What is the temperature when the volume is reduced to 9.0 dm3 at the same pressure?
A. −3.0 °C
B. 24.3 °C
C. 29.7 °C
D. 57.0 °C
Markscheme
A
Examiners report
What is the coefficient of (aq) when the equation is balanced using the smallest possible whole numbers?
A. 1
B. 2
C. 3
D. 4
Markscheme
B
Examiners report
How many moles of FeS2 are required to produce 32 g of SO2? (Ar: S = 32, O = 16)
4FeS2 (s) + 11O2 (g) → 2Fe2O3 (s) + 8SO2 (g)
A. 0.25
B. 0.50
C. 1.0
D. 2.0
Markscheme
A
Examiners report
An antacid tablet containing 0.50 g of NaHCO3 (Mr = 84) is dissolved in water to give a volume of 250 cm3. What is the concentration, in mol dm−3, of HCO3− in this solution?
A.
B.
C.
D.
Markscheme
B
Examiners report
Which combination is correct?
Markscheme
B
Examiners report
Which electron transition emits energy of the longest wavelength?
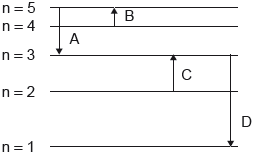
Markscheme
A
Examiners report
What is the number of carbon atoms in of ethanoic acid , ?
A.
B.
C.
D.
Markscheme
D
Examiners report
Approximately 57% of candidates could correctly apply Avogadro's number in calculating number of carbon atoms in 12 g of ethanoic acid. Many candidates did not identify that there were 2 carbon atoms per molecule.
How many atoms of nitrogen are there in 0.50 mol of (NH4)2CO3?
A. 1
B. 2
C. 3.01 × 1023
D. 6.02 × 1023
Markscheme
D
Examiners report
What is the percentage yield when 2.0 g of ethene, C2H4, is formed from 5.0 g of ethanol, C2H5OH?
Mr(ethene) = 28; Mr(ethanol) = 46
A.
B.
C.
D.
Markscheme
B
Examiners report
The complete combustion of 15.0cm3 of a gaseous hydrocarbon X produces 60.0 cm3 of carbon dioxide gas and 75.0 cm3 of water vapour. What is the molecular formula of X? (All volumes are measured at the same temperature and pressure.)
A. C4H6
B. C4H8
C. C4H10
D. C6H10
Markscheme
C
Examiners report
5.0mol of Fe2O3(s) and 6.0mol of CO(g) react according to the equation below. What is the limiting reactant and how many moles of the excess reactant remain unreacted?
Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO2(g)
Markscheme
B
Examiners report
0.10 mol of hydrochloric acid is mixed with 0.10 mol of calcium carbonate.
2HCl (aq) + CaCO3 (s) → CaCl2 (aq) + H2O (l) + CO2 (g)
Which is correct?
Markscheme
C
Examiners report
Which graph shows the relationship between the volume and pressure of a fixed mass of an ideal gas?
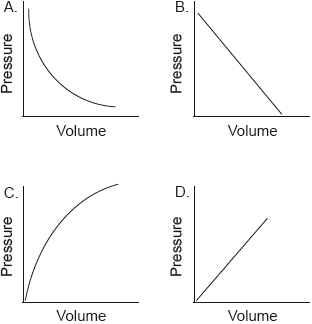
Markscheme
A
Examiners report
Which graph represents the relationship between the amount of gas, n, and the absolute temperature, T, with all other variables in the ideal gas equation, PV = nRT, held constant?
Markscheme
B
Examiners report
56% of the candidates selected the correct graph representing the relationship between the amount of gas and its absolute temperature. The most commonly chosen distractor gave a directly proportional relationship.